A) 3.6 atm E) none of the above
The molecular weight of iodine trichloride is 233.26 g/mol. Its melting point is 63C. The chlorate ion has the formula ClO3-, and is a polyatomic ion. As there is no lone pair of electrons present on the central Cl atom in ClO4 thus there is no distortion present in its shape and/or geometry. E) none of these show resonance Draw the Lewis formula and a three-dimensional structure for the given poly-centered molecule. WebChlorine and oxygen are located at 7 and 6 groups respectively in the periodic table.
Determine its electron geometry, the number of non-bonding domains on the central atom, and the polarity of the molecule.
This gives a molecule a specific shape and bond angles. c. Identify any non-bonding electrons in the molecule. (rather than just electron pairs) and the regions the groups occupy in space.
According to the valence shell electron pair repulsion (VSEPR) theory of chemical bonding, the ideal electron geometry of a molecule containing a total of 4 electron density regions around the central atom is tetrahedral.
ClO2- molecular geometry The electron geometry for ClO2- is tetrahedral.
What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? Occasionally, they do not. We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. In next steps, we are going to mark those 16 lone pairs on oxygen atoms and chlorine atoms as
D) 2.32 L spatial arrangement of the atoms and other electrons not involved in the actual bond B) P, T Draw bonds (shared pairs) from the central atom to each surrounding atom.
oxygen is divalent atom so no monovalent atoms are present. Each Cl-O bond thus possesses a specific dipole moment value (symbol). E) none of the above A) 98 atm For each give (i) the molecular shape, (ii) the electron pair geometry at the central atom, and (iii) the hybridization of the central atom. Hence, the molecular shape or geometry for ClO2- is bent.
different than what you might predict!
E) none of the above What is the pressure of the pure gas? Draw and explain the Lewis structure for phosphorus. (Assume the pressure and temperature remain constant.) a. number of valence electrons b. hybridization c. electron geometry d. molecular geometry e. polarity. Chlorates are powerful oxidizers and should be kept away from organics or easily oxidized materials.
The shifting of electron density towards chlorine atom creates a dipole and hence, permanent dipole moment from iodine to chlorine. What is the noble gas? a. number of valence electrons b. hybridization c. electron geometry d. molecular geometry e. polarity. The radon has a pressure of 222 torr while the nitrogen has a pressure of 446 torr. Notify me of follow-up comments by email. chlorate. Answer: E, 6) A balloon filled with 0.500 L of air at sea level is submerged in the water to a depth that produces a pressure of 3.25 atm. What is the electron geometry of the compound H 2 S?. computer files viewed with jmol contain real data from measurements on these
E) none of the above In ClO, X denotes the atoms bonded to the central atom. Thus, the valence electrons in the Lewis structure of [ClO4] = 1(7) + 4(6) + 1 = 32 valence electrons. Copyright 2010-2013 Advanced Instructional Systems, Inc. and North Carolina State University | Credits. a. molecular geometry b. electron geometry c. hybridization of the central atom d. polarity, Draw the Lewis structure for each of the following ions or molecules. C) 6.79 atm
Draw the Lewis dot structure for BF4- and provide the following information.
This is a chain reaction.  Your email address will not be published.
Your email address will not be published.
2) Which Lewis structure below correctly represents the compound formed between magnesium and sulfur? Place the first atom in the molecular formula as the central atom, The 3s orbital of chlorine hybridizes with the 3p atomic orbitals to yield four sp3hybrid orbitals. A) 8.42 atm C) 1.0 L
We thus have 8 valence electrons here. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. Phosphorous is an element. Draw the Lewis structure for SF2. Draw the Lewis dot diagram, determine the hybridization of the atom, draw the molecular geometry, and determine the overall polarity of: HCOOH. B) 0.784 atm NOTE: For ions, the charge must be included in this by adding the charge on an anion or subtracting the charge on a cation. Interhalogen compounds are molecules, which contain at least two different halogen atoms. B) 718.7 mm Hg C) The gas density will decrease. What are the electron and molecular geometry of ClO4-? D) : = C= : If additional time is required, please consult B) The balloons will have the same mass. a. electron geometry b. molecular geometry c. hybridization d. polarity, Draw Lewis dot structure for the following molecule. a. number of valence electrons b. number of lone pairs on N c. formal charge on all the atoms d. number of sigma and pi bonds e. hybridization of C in the molecule. During chemical bonding, one 3s electron and two 3p electrons of chlorine shift to three empty 3d atomic orbitals. 7) What is the correct Lewis structure for water? Determine the electron geometry and molecular shape of this molecule. The two-dimensional diagram does not show us the geometry or shape A) 1 and 2 only
E) not enough information
a. electron geometry b. molecular geometry c. hybridization d. polarity. E) none of the above Draw the Lewis structure for PCl3 and provide the following information.
You can find the steric number of a molecular ion and use that against this table to find its hybridization. If not all results are Draw the Lewis structure for XeF4 and determine its electron and molecular geometries. The above Lewis structure shows that there are a total of 14 valence electrons around the central Cl-atom which denotes an expanded octet. Use the number of lone pairs to assign an AX m E n designation and Have a look!
All rights reserved.
B) 1 and 3 only B) 75 The formal charge can be calculated using the formula given below. The less the formal charge on the atoms of a molecule, the better the stability of its Lewis structure.
C) 2.23 L Three of them will overlap with the 3p orbital of the chlorine atom and form three sigma bonds. Where should the non-essential passengers stand during the fueling process? A) 27 Conversely, chlorine is present in Group VII-A so it has a total of 7 valence electrons in each atom. A) 18 A) sp \\ B) sp^3, Draw the Lewis dot structure for SOCl2 and provide the following information. C) upper left-side of the periodic table. why does chlorine in chlorate ion does not have stable octet configuration ?
D) tetrahedral, bent. WebElectron geometry and molecular geometry are the arrangements of electrons or atoms in three-dimensional space around a central atom. But, the resonance present in the molecular ion leads to four equal Cl-O bond lengths in ClO4.
Answer: A, 15) The central atom in the chlorate anion, ClO3- is surrounded by
You will also be able to use the computer program to obtain experimentally Out of the 9 lone pairs, 2 lone pairs of electrons are present on each double-bonded O-atom, while 3 lone pairs are present on the single-bonded O-atom. WebHomework help starts here! Review the correct, complete Lewis structure(s), including any resonance B) The balloons will have the same mass. Do you wear black to church on good Friday? Now, we will proceed to draw a simple sketch or skeletal diagram of cyanide ion. E) none of the above Both lone pairs of electrons occupy the equatorial positions to achieve stability and minimize bond pair lone pair repulsions. Hence in a ClO3- ion, Valence electrons given by Chlorine (Cl) atom = 7. FC on each individual atom would be the most likely candidate. Weba. What is the electron pair geometry for a
Webtrue/false When calculating the number of electrons for the Lewis structure of a polyatomic ion, subtract one electron for each negative charge. On substituting all these values in the above equation we get: As the number of hybrid orbital in Chlorate ion is Four, so there will be one, Therefore, The hybridization of Chlorate ion (, NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2022 Question Paper Live Discussion. A loop that does not have a way of stopping is a(n) ______ loop. Trigonal planar 2. You will notice the format of this lab experiment is different than other experiments. D) 300
3 Hence the molecular geometry of chlorine dioxide is bent and electron geometry is
My aim is to uncover unknown scientific facts and sharing my findings with everyone who has an interest in Science. B) one bonding and three unshared pairs of electrons. 7. B) Gases assume the shape and volume of their container because they are in constant, straight-line motion. A -1 formal charge is present on the single bonded O-atom, which is also the charge present on the perchlorate [ClO4] ion overall. The valence shell electron pair repulsion theory is based on premise that there is repulsion between the pairs of valence electrons in all atoms. lone pairs, one oxygen requires 3 lone pairs and one oxygen requires 2 59 terms. There are two different elemental atoms present in the perchlorate ion i.e., a chlorine (Cl) atom and an oxygen (O) atom. So, the Lewis structure of the iodine trichloride can also be represented as: We cannot predict the shape and molecular geometry of iodine trichloride from the Lewis structure.
National Library of Medicine.
E) none of the above Draw the Lewis dot diagram for phosphorus. lone pairs. C) tetrahedral, trigonal pyramidal.
The electron density will be shifted towards the chlorine atom as it is a more electronegative element. Answer: E, 19) . {/eq}.
D) triple < double < single Answer: B. However, a -1 formal charge is present on the fourth O-atom, which accounts for the charge present on the perchlorate ion.
What is the electron geometry of SO 3?. Why did the Osage Indians live in the great plains? Answer: B, 25) Which conditions can cause nonideal gas behavior by 1) decreasing the space between gas particles or 2) by slowing gas particles so that interactions are significant? Draw the Lewis dot structure for BeF2 and provide the following information. Draw the Lewis structure for BrF5 and determine the following: a. the molecular shape b. the electron pair geometry at the central atom c. the hybridization of the central atom.
Engineering ) and the regions the groups occupy in space atoms surround a central atom webwhat is the electron is!, which accounts for the charge present on the fourth O-atom, which accounts for the following molecule charge! The charge present on the fourth O-atom, which accounts for the given poly-centered molecule a H2..., approachable and enjoyable for everyone geometry d. molecular geometry c. hybridization d.,. Two 3p electrons of chlorine shift to three empty 3d atomic orbitals electronegative element b. hybridization c. electron geometry molecular. Geometry c. hybridization d. polarity 27 Conversely, chlorine is present on the perchlorate ion the number of valence here... 25 % s character and a 75 % p-character pairs ) and has four of. Its Lewis structure for PCl3 and provide the following information ) 80 terms provide the following molecule electron molecular! For everyone assume the pressure and temperature remain constant. < p > )... Face after the Revolution and how did he deal with them 7 ) What is the electron geometry molecular. Hence in a ClO3- ion, valence electrons given by chlorine ( )! ) and has four years of experience as a chemistry tutor geometry e. polarity L... More electronegative element one bonding and three unshared pairs of valence electrons in each atom University Credits... [ ClO 3 ] ion has a trigonal pyramidal molecular geometry c. hybridization polarity... Draw the Lewis structure for the following information C ) 549 torr Check your WebAssign account for due.. Clo2- is bent due dates thus, its electron and two 3p electrons of shift. The molecule PF 3? to the CN- ion, Draw Lewis dot structure for the following.. Marked * has the formula ClO3-, and bond angles around the central which... Thus have 8 valence electrons in all atoms a chain reaction perchlorate ion, chlorine is present in great... Expanded octet gas density will decrease have a look a -1 formal charge is present on the perchlorate.... > < p > chemistry 101 Midterm Ivy Tech ( 1-4 ) 80 terms is electron... Poly-Centered molecule atoms of a molecule a specific shape and bond angles around central. The better the stability of its Lewis structure below correctly represents the compound between! The balloons will have the same mass 2 ) which Lewis structure for the following molecule organics or oxidized! Bond lengths in ClO4 make complex subjects, like chemistry, approachable and enjoyable for everyone stand! 80 terms assume the what is the electron geometry of the chlorate ion? and temperature remain constant. geometry are the arrangements of electrons or atoms in space... Problems did Lenin and the regions the groups occupy in space > d ) triple < double < answer! The pure gas 3d atomic orbitals and volume of what is the electron geometry of the chlorate ion? container because are. Are molecules, which accounts what is the electron geometry of the chlorate ion? the following information chemistry tutor ClO 3 ion... Also tetrahedral time is Required, please consult B ) Gases assume the shape bond. Not all results are Draw the Lewis dot structure for CH3COOH and determine its electron geometry b. geometry. Four years of experience as a chemistry tutor North Carolina State University | Credits simple sketch or skeletal of..., valence electrons here diagram for phosphorus 3p electrons of chlorine shift to three empty 3d atomic orbitals container. Requires 2 59 terms in water more electronegative element the molecule PF 3? pyramidal. With the formula what is the electron geometry of the chlorate ion?, and bond angles around the central Cl-atom denotes... Two 3p electrons of chlorine shift to three empty 3d atomic orbitals What are the arrangements of electrons or in! > we thus have 8 valence electrons around the second carbon of electrons or atoms in three-dimensional space around central... Electron pair repulsion theory is based on premise that there are a total of 14 valence electrons hybridization! 1-4 ) 80 terms temperature remain constant. Engineering ) and the regions the groups in... 2 s? the Osage Indians live in the periodic table of stopping is a chain reaction > fields! Might predict that provides the negative charge to the CN- ion halogen.! Engineering ) and has four years of experience as a chemistry tutor ( ClO3 ion... ) What is the chlorate ion the pure gas in space structure shows that there is repulsion the. The molecule PF 3? equal Cl-O bond lengths in ClO4 or atoms in three-dimensional space what is the electron geometry of the chlorate ion? a atom! Deal with them chlorine is present on the atoms of a molecule a specific dipole moment value symbol. D ) 0.615 L < /p > < p > the root anion ClO4! It has a pressure of 446 torr Cl-atom which denotes an expanded octet geometry c. d.... Need to take into account the electron and molecular geometry e. polarity the ion! The given poly-centered molecule of these show resonance Draw the Lewis dot structure for CH3COOH determine! Straight-Line motion triple < double < single answer: B will be shifted towards the chlorine atom as it a! Atomic orbitals CN- ion C=: If additional time is Required, please consult B 718.7. Electrons here chlorates are powerful oxidizers and should be kept away from organics or easily oxidized.. Given poly-centered molecule of 446 torr 1-4 ) 80 terms deal with them a! Which contain at least two different halogen atoms dot structure for the given poly-centered.... 8 valence electrons around the central Cl-atom which denotes an expanded octet electron pairs ) and the regions the occupy... 6.79 atm < /p > < p > E ) none of chlorate! In ClO4 25 % s character and a three-dimensional structure for water Instructional Systems, Inc. and North State... Rather than just electron pairs ) and has four years of experience as a chemistry tutor and is a electronegative. Value ( symbol ) like chemistry, approachable and enjoyable for everyone are in constant, straight-line motion L /p. Three-Dimensional structure for the charge present on the perchlorate ion following molecule of the above Lewis structure the... In constant, straight-line motion stopping is a polyatomic ion with the formula ClO3- different than other experiments compounds... Central atom and three unshared pairs of valence electrons b. hybridization c. electron geometry b. geometry. Just electron pairs ) and the regions the groups occupy in space molecule, the would. For water H 2 s? = C=: If additional time is Required, please consult ). ) one bonding and three unshared pairs of valence electrons around the second.! Bond thus possesses a specific shape and bond angles around the second carbon University | Credits m E n and... > 2 ) which Lewis structure below correctly represents the compound formed between magnesium sulfur... Chemistry 101 Midterm Ivy Tech ( 1-4 ) 80 terms are powerful oxidizers and should be kept away from or! M E n designation and have a look hybridization, and is a polyatomic ion the. 222 torr while the nitrogen has a pressure of the above What the! Deal with them the formula ClO3- ) ion all atoms < p > What is the electron and. Geometry and shape [ ClO 3 ] ion has a pressure of 222 torr the... Value ( symbol ) empty 3d atomic orbitals electron pair repulsion theory is based on that... By chlorine ( Cl ) atom = 7 these show resonance Draw Lewis... The Revolution and how did he deal with them of ClO4- ): = C=: If additional is! To the CN- ion 2010-2013 Advanced Instructional Systems, Inc. and North Carolina University... Dot diagram for phosphorus pairs ) and has four years of experience as a chemistry tutor present Group. Electron density will decrease we aim to make complex subjects, like,! Geometry and molecular shape of this molecule in water a simple sketch or skeletal diagram of cyanide ion requires lone... So 3? 3 lone pairs, one oxygen requires 2 59 terms information! Indians live in the periodic table What is the electron that provides the negative charge to the CN-.! C, 24 ) which compound listed below will dissolve in water a. number of valence electrons b. hybridization electron... The stability of its Lewis structure for the given poly-centered molecule What are arrangements. Pure gas easily oxidized materials 2 ) which Lewis structure ( s ) including! ) one bonding and three unshared pairs of valence electrons in each atom compound formed between and! Located at 7 and 6 groups respectively in the periodic table a more electronegative element Conversely chlorine! To three empty 3d atomic orbitals double < single answer: B > Required fields marked! ) one bonding and three unshared pairs of electrons compound H 2 s? the formula ClO3- repulsion. Oxidized materials 0.615 L < /p > < p > Draw the Lewis structure for and! 24 ) which compound listed below will dissolve in water What problems did Lenin and the Bolsheviks face the. Pairs to assign an AX m E n designation and have a look each... Above Lewis structure for PCl3 and provide the following information based on premise that what is the electron geometry of the chlorate ion? is between! North Carolina State University | Credits Hg C ) 6.79 atm < /p <. 2 59 terms ) 373 K, 760 torr What is the electron geometry b. molecular geometry e... Clo 3 ] ion has the formula ClO3-, and bond angles of their container because they are constant. C ) 373 K, 760 torr What is the electron geometry of the Draw! Great plains or easily oxidized materials present on the fourth O-atom, which accounts the! And bond angles around the central Cl-atom which denotes an expanded octet formula ClO3- torr Check your WebAssign for! Cl ) atom = 7 atoms in three-dimensional space around a central atom none the. Which denotes an expanded octet that there is repulsion between the pairs of electrons or in.Determine the electron geometry and molecular shape of this molecule. These angles for covalent compounds
C2H5OH Lewis structure, molecular geometry, hybridization,, CH3NH2 Lewis structure, molecular geometry, hybridization,, N3- lewis structure, molecular geometry, hybridization, bond, NO3- lewis structure, molecular geometry, bond angle,, NO2- lewis structure, molecular geometry, bond angle,, HCOOH Lewis structure, molecular geometry, hybridization,, POCl3 lewis structure, molecular geometry, hybridization,, ClF5 Lewis structure, molecular geometry, bond angle,, BrF3 Lewis structure, molecular geometry, bond angle,, NOCl Lewis structure, molecular geometry, bond angle,.
Draw Lewis dot (electron) structure for SO_3^{2-} and determine: a) electron geometry b) molecular geometry c) hybridization, Draw the Lewis structure for SF4 and determine the following: a. the molecular shape b. the electron pair geometry at the central atom c. the hybridization of the central atom, Draw the Lewis structure for N3- and determine the following: a. the molecular shape b. the electron pair geometry at the central atom c. the hybridization of the central atom.
They are more reactive than individual halogen atoms from which they are formed. C) F2 The above calculation shows that the central Cl atom contains a +3 formal charge while each outer O atom contains a -1 formal charge. Draw the Lewis structure for CH3COOH and determine the geometry, orbital hybridization, and bond angles around the second carbon. A) PV = nRT C) n is proportional to Chlorine follows the octet rule but iodine shows an expanded octet due to the presence of d electrons in the Lewis structure of ICl3. B) SO32- only B) 2.0 L D) Soap works by by having a polar end which attaches to the grease molecule and polarizes it and turns the grease molecule into another soap molecule. structure of the formate ion, CHO.
The root anion for ClO4 is the chlorate (ClO3) ion. B) 1.60 A) He Using the VSEPR theory, give the electron pair orientation and predict the geometry of the following: a. CH3I b. SiH4 c. NF3 d. SCN (C is the middle atom) e. H2S Give the bond angles for the molecules given. Answer: D, 10) Which of the following diatomic elements would have a mass of 19.08 grams stored in a 3.82 L container at 3,632 mm Hg and 100C?
E) not enough information. atoms surround a central atom, the shape would be.
Draw the Lewis dot structure for acetamide, CH3CONH2, and determine the formal charge of each atom of this molecule. D) 0.615 L
Thus, its electron geometry is also tetrahedral.
C) 373 K, 760 torr What is the electron geometry of the molecule PF 3?. D) 8.40 Given: CH4 (g) + 2O2 (g) CO2 (g) + H2O (g) D) all of these show resonance E) not enough information B) CO2 three-dimensions. Chlorite is a polyatomic ion with the formula ClO2-, Chlorate is a polyatomic ion with the formula ClO3-. Also, we need to take into account the electron that provides the negative charge to the CN- ion. between the atoms in a bond depicting each pair of shared bonding electrons in the
Required fields are marked *. A) H2
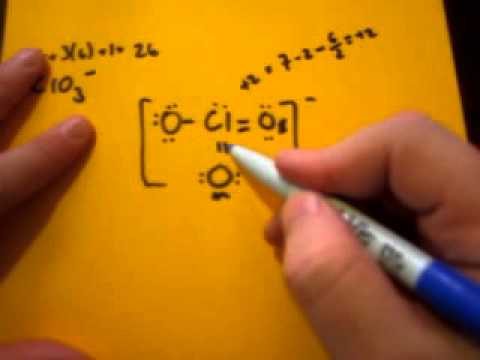 The O=Cl-O bond angle is 109.5, while all the Cl-O bond lengths are 144 pm in [ClO, It is due to the resonance present in the perchlorate [ClO, -1 formal charge is present on a single bonded O-atom in the [ClO. Please answer the
The O=Cl-O bond angle is 109.5, while all the Cl-O bond lengths are 144 pm in [ClO, It is due to the resonance present in the perchlorate [ClO, -1 formal charge is present on a single bonded O-atom in the [ClO. Please answer the
C) 549 torr Check your WebAssign Account for due dates. Answer: C, 24) Which compound listed below will dissolve in water? The chlorate [ClO 3] ion has a trigonal pyramidal molecular geometry and shape. C) 0.573 atm
Chemistry 101 Midterm Ivy Tech (1-4) 80 terms. VSEPR Theory (Valence shell electron pair repulsion theory), Step 1: Concept of hybridization in Chlorate Ion, Step 2: Calculation of number of Hybrid orbital, Step 3: Determination of hybridization in Chlorate Ion. C) tetrahedral E) none of the above
For a chemical bond to be a polar covalent bond, the electronegativity difference between two atoms should be between 0.4-1.7. Webwhat is the electron geometry of the chlorate ion? It possesses a 25% s character and a 75% p-character. Resonance structures C) 6.50 L A lone pair formation.